The 2025 Nobel Prize in Medicine will be awarded to three researchers who discovered the mechanism of peripheral immune tolerance, which prevents the immune system’s T cells from attacking the body’s own cells. The prize will be shared equally by Mary Brunkow of the Institute for Systems Biology in Seattle, Fred Ramsdell of Sonoma Biotherapeutics in California, and Shimon Sakaguchi of Osaka University in Japan.
Nobel Prize in Physiology or Medicine 2025: Peripheral Immune Tolerance
A New Type of Cell
Our immune system can recognize thousands of disease-causing agents, as well as foreign substances such as allergens, and mount an attack against them. Occasionally, however, it mistakenly targets the body’s own self-components—a condition known as an autoimmune disease. To prevent this, a safeguard mechanism develops early in life during the formation of the immune system. The primary mechanism, which operates in the thymus and bone marrow where T and B cells mature, eliminates cells that could act against self-components of the body.
The three Nobel laureates discovered that, in addition to this mechanism, a secondary mechanism exists—one that “catches” the self-reactive cells that manage to escape the first line of defense.
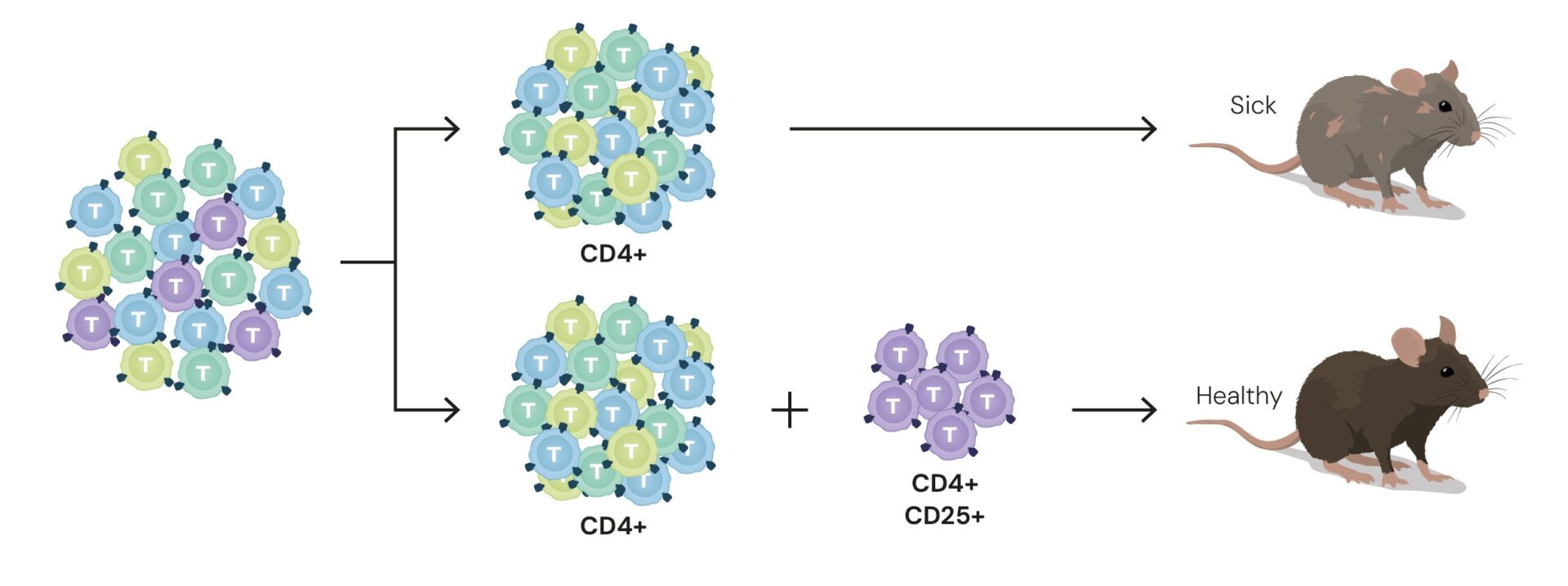
Shimon Sakaguchi, born in 1951 in Shiga, Japan, studied at Kyoto University and later became a professor at Osaka University. In the 1980s, through experiments in mice, he identified a new type of T cell. From the total T-cell population, he succeeded in isolating only those cells producing specific proteins—CD4 and CD25, associated with the major histocompatibility complex, which helps distinguish self from non-self. When he genetically engineered mice whose T cells could not produce the CD4 and CD25 proteins, the mice developed autoimmune diseases. Conversely, when he injected CD4⁺CD25⁺ T cells into mice with autoimmune conditions that lacked this subset of T cells, their condition improved. In other words, these cells suppressed the immune response.
Today, the subset of T cells that express the CD4 and CD25 proteins (CD4⁺CD25⁺ cells) is known as regulatory T cells (Tregs). These cells represent only about 10 percent of the total T-cell population.
From Radioactive Mice to Autoimmune Diseases
Mary Brunkow, born in 1961, studied at Princeton University in New Jersey, USA. Fred Ramsdell, born in 1960 in Illinois, completed his PhD at the University of California in 1987. Their paths converged when they worked together at Celltech Chiroscience, a company developing drugs for autoimmune diseases.
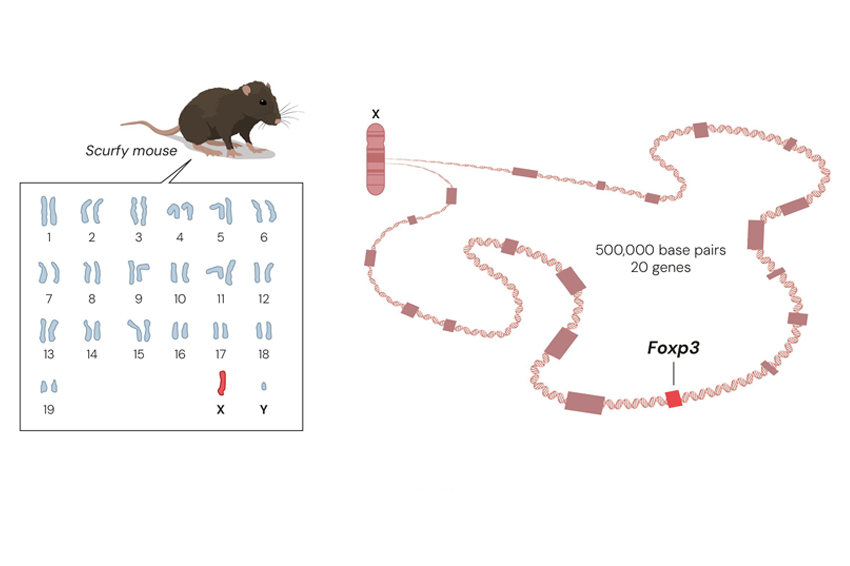
The two studied a mouse strain known as scurfy, first identified during the Manhattan Project in experiments on radiation exposure. Only male mice exhibited the syndrome—characterized by scaly, dry skin—and survived for just a few weeks after birth. Because the defect appeared exclusively in males, researchers concluded that the responsible gene lay on the X chromosome. Males possess only one X chromosome, so a single faulty copy was sufficient to cause disease, whereas females, with two copies, were protected by the healthy one. Still, the identity and function of the defective gene remained unknown.
Brunkow and Ramsdell hypothesized that scurfy mice suffered from a genetic autoimmune disease, and that uncovering its mechanism might aid in developing new treatments. They mapped the defective region of the X chromosome and identified twenty candidate genes. By comparing these genes in scurfy and healthy mice, they discovered that the defective gene belonged to the FOX family, a group of genes that regulate the activity of other genes and thereby influence cell development.
They suspected that children with a rare autoimmune disorder called IPEX syndrome might carry a defect in the same gene. Indeed, patient samples revealed mutations in the FOXP3 gene. Their findings were published in three papers (1, 2, and 3) in Nature Genetics in 2001.
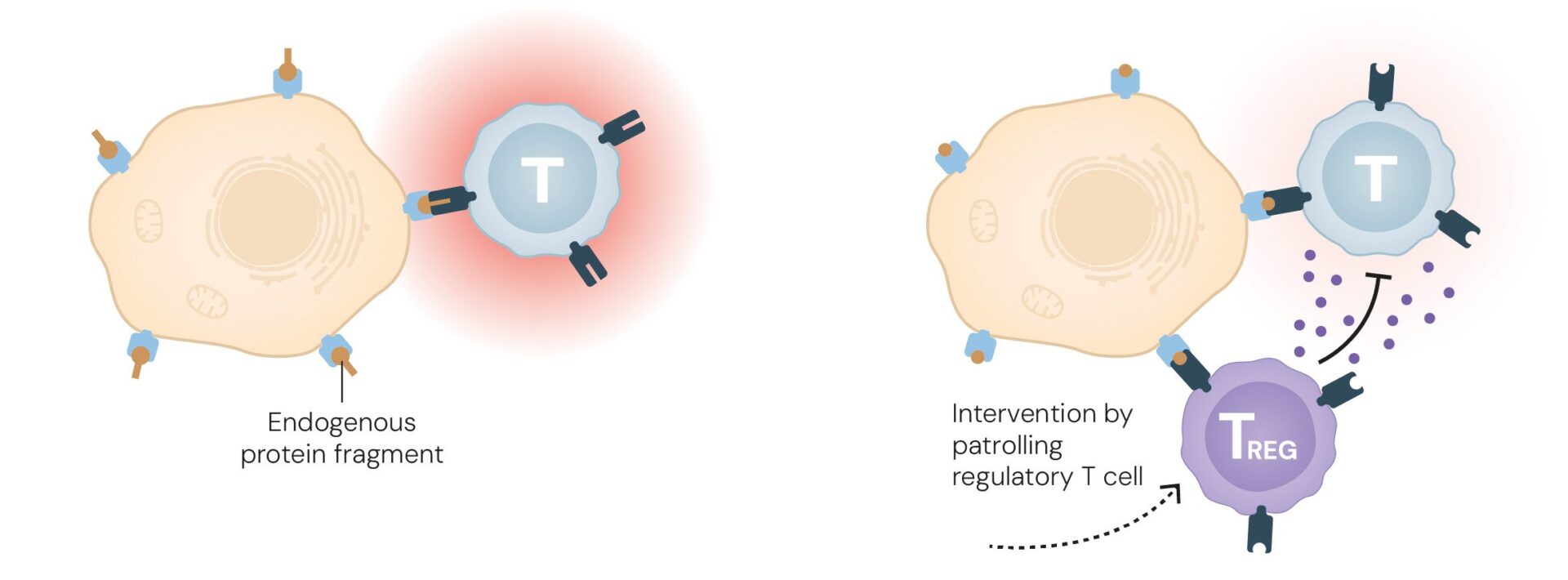
Two years later, Sakaguchi linked the FOXP3 gene to the regulatory T cells (Tregs) he had discovered years earlier, and realized the gene’s crucial role in enabling these cells to prevent autoimmune diseases. When FOXP3 is defective, regulatory T cells fail to develop properly.
Medical Applications
These discoveries spurred laboratories around the world to investigate the connection between regulatory T cells, the FOXP3 gene, and autoimmune diseases, paving the way for the development of treatments not only for these conditions but also for cancer and other immune-related disorders.
Like other T cells, regulatory T cells are produced in the thymus. Ongoing research is investigating their potential to suppress immune responses in organ transplant recipients and patients with autoimmune diseases. Conversely, scientists are also exploring ways to harness the ability of these cells to stimulate and activate the immune system in individuals with cancerous tumors. In this context, the aim is to direct the immune system against specific body cells—the tumor cells—so it can attack and eliminate them, helping patients overcome the disease.
This year, each Nobel Prize carries a monetary award of 11 million Swedish kronor, equivalent to roughly 1.17 million US dollars. The prizes will be formally presented on December 10, the anniversary of Alfred Nobel’s death.
Announcement of the 2025 Nobel Prize in Physiology or Medicine:
Additional content that may interest you
Searching for Water on the Moon: This Week in Space
A private spacecraft and a NASA satellite are en route to the Moon, an asteroid impact risk has been ruled out, Starship is preparing for another test flight, and Katy Perry is set to journey to the edge of space. This Week in Space.


